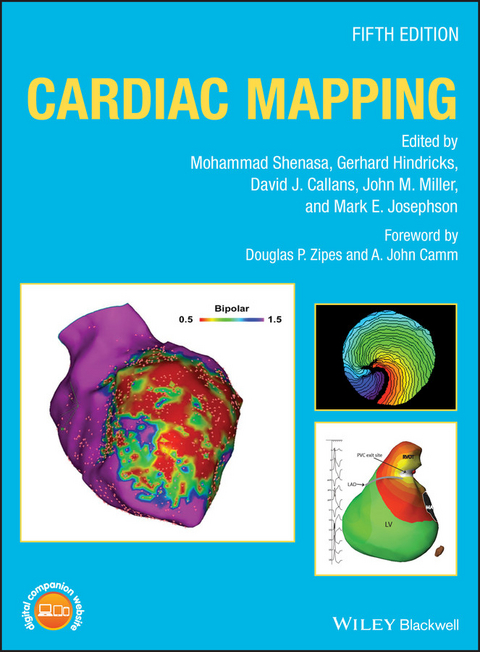
Cardiac Mapping (eBook)
John Wiley & Sons (Verlag)
978-1-119-15261-3 (ISBN)
The expanded guide to cardiac mapping
The effective diagnosis and treatment of heart disease may vitally depend upon accurate and detailed cardiac mapping. However, in an era of rapid technological advancement, medical professionals can encounter difficulties maintaining an up-to-date knowledge of current methods. This fifth edition of the much-admired Cardiac Mapping is, therefore, essential, offering a level of cutting-edge insight that is unmatched in its scope and depth.
Featuring contributions from a global team of electrophysiologists, the book builds upon previous editions' comprehensive explanations of the mapping, imaging, and ablation of the heart. Nearly 100 chapters provide fascinating accounts of topics ranging from the mapping of supraventricular and ventriculararrhythmias, to compelling extrapolations of how the field might develop in the years to come. In this text, readers will find:
- Full coverage of all aspects of cardiac mapping, and imaging
- Explorations of mapping in experimental models of arrhythmias
- Examples of new catheter-based techniques
- Access to a companion website featuring additional content and illustrative video clips
Cardiac Mapping is an indispensable resource for scientists, clinical electrophysiologists, cardiologists, and all physicians who care for patients with cardiac arrhythmias.
Mohammad Shenasa, MD, PhD, Attending Physician, Department of Cardiovascular Services, O'Connor Hospital, Heart & Rhythm Medical Group, San Jose, CA, USA.
Gerhard Hindricks, MD, Professor of Medicine (Cardiology), University of Leipzig, Heart Center Director, Department of Electrophysiology, Leipzig, Germany.
David J. Callans, MD, Professor of Medicine, Cardiovascular Division, Department of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
John M. Miller, MD, Professor of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA.
Mark E. Josephson, MD, Professor Emeritus, Herman C. Dana Professor of Medicine, Harvard Medical School, Chief of Cardiovascular Division, Beth Israel Deaconess Medical Center, Boston, MA, USA.
The expanded guide to cardiac mappingThe effective diagnosis and treatment of heart disease may vitally depend upon accurate and detailed cardiac mapping. However, in an era of rapid technological advancement, medical professionals can encounter difficulties maintaining an up-to-date knowledge of current methods. This fifth edition of the much-admired Cardiac Mapping is, therefore, essential, offering a level of cutting-edge insight that is unmatched in its scope and depth.Featuring contributions from a global team of electrophysiologists, the book builds upon previous editions' comprehensive explanations of the mapping, imaging, and ablation of the heart. Nearly 100 chapters provide fascinating accounts of topics ranging from the mapping of supraventricular and ventriculararrhythmias, to compelling extrapolations of how the field might develop in the years to come. In this text, readers will find: Full coverage of all aspects of cardiac mapping, and imaging Explorations of mapping in experimental models of arrhythmias Examples of new catheter-based techniques Access to a companion website featuring additional content and illustrative video clips Cardiac Mapping is an indispensable resource for scientists, clinical electrophysiologists, cardiologists, and all physicians who care for patients with cardiac arrhythmias.
Mohammad Shenasa, MD, PhD, Attending Physician, Department of Cardiovascular Services, O'Connor Hospital, Heart & Rhythm Medical Group, San Jose, CA, USA. Gerhard Hindricks, MD, Professor of Medicine (Cardiology), University of Leipzig, Heart Center Director, Department of Electrophysiology, Leipzig, Germany. David J. Callans, MD, Professor of Medicine, Cardiovascular Division, Department of Medicine, University of Pennsylvania, Philadelphia, PA, USA. John M. Miller, MD, Professor of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA. Mark E. Josephson, MD, Professor Emeritus, Herman C. Dana Professor of Medicine, Harvard Medical School, Chief of Cardiovascular Division, Beth Israel Deaconess Medical Center, Boston, MA, USA.
List of Videos
Video clip 8.1 Dense mapping in the suspected area of flutter allowed understanding of a small reentrant circuit in the left atrial roof close to the left superior pulmonary vein. Map showing activation in atrial flutter from superior and posterior view.
Video clip 8.2 Right and left atrial map in LAO view showing mitral isthmus flutter in a patient with prior cavotricuspid isthmus ablation. The entire tachcyardia cycle length can be noted also in the right atrium coincidentally.
Video clip 13.1 Optogenetics pacing of the isolate Langendorff‐perfused rat heart preparation. As can be seen, the application of flashes of blue light to the site of ChR2 transgene delivery (apex) resulted in efficient capture of the ventricle. Note the increase in the contraction rate from ~75 bpm at baseline to 200 bpm during application of flashes of blue light at the same frequency.
Video clip 15.1 Assessment of pulmonary vein anatomy with MDCT prior to RFCA. Analysis of the MDCT axial images is the first step to evaluate pulmonary vein anatomy. Scrolling through them in the caudal to cranial direction as this video shows, we can identify first the left and right inferior pulmonary veins draining into the left atrium, the right superior pulmonary vein, the left atrial appendage, and finally the left superior pulmonary vein.
Video clip 15.2 Evaluation of left ventricular arrhythmogenic substrate with MRI. The left ventricular two‐chamber view shows a large, thinned, and akinetic anteroapical wall that corresponds to a prior anterior myocardial infarction. On contrast‐enhanced MRI, this area will appear as an extensive bright, hyperenhanced area.
Video clip 25.1 Simultaneous optical mapping of the posterior wall and the appendage of the left atrium (PLA/LAA) with two synchronized CCD cameras. Time series of voltage‐sensitive fluorescence were transformed to the phase domain and color‐coded according to the stages of the action potential (wavefront is the blue–purple boundary). The phase movies show a rotor with its center of rotation (a phase singularity point representing the surface end point of a filament perpendicular to the field of view) drifting towards the LAA (right). As soon as the rotor enters in the field of view of the LAA, the patterns of activation switch from breakthroughs to reentry and the meandering rotor becomes the main source driving the AF at DFMax. Similarly, once the rotor travels outside of the field of view, the pattern of activation in the LAA switches back to breakthroughs and DF decreases.
Video clip 25.2 Spiral wave reentries (rotors) initiated by S1–S2 cross‐field protocol in two‐dimensional computer models of paroxysmal (left) and paroxysmal‐to‐persistent transition (right) AF. Paroxysmal AF rotors exhibited lower frequency than that in transition AF. Rotors in paroxysmal AF meandered considerably and eventually self‐terminated on collision with boundary. In transition AF, the rotors were more stable, had higher frequency, and persisted throughout the simulation.
Video clip 31.1 Figure‐of‐eight reentrant ventricular tachycardia in the anterior septal wall of a swine with chronic anterior wall infarction.
Video clip 33.1 Chaotic Ca2+ dynamics at subcellular resolution during VF.
Video clip 40.1 (a) A Carto™ three‐dimensional propagation map (derived from electroanatomical mapping) during typical or counterclockwise CTI‐dependent AFL. Note the counterclockwise activation sequence around the tricuspid valve annulus. (b) A Carto 3™ three‐dimensional propagation map (derived from electroanatomical mapping) during reverse typical or clockwise CTI‐dependent AFL. Note the clockwise activation sequence around the tricuspid valve annulus. The red circles indicate the area in the CTI ablated after creating the propagation/activation map.
Video clip 40.2 A Velocity™ three‐dimensional propagation map (derived from electroanatomical mapping) during typical AFL. Note the clockwise activation sequence around the tricuspid valve annulus.
Video clip 40.3 A Velocity™ three‐dimensional propagation map (derived from electroanatomical mapping) following CTI ablation during pacing from the proximal coronary sinus. Note the conduction block from medial to lateral at the line of ablation in the CTI. Lateral to medial conduction block was also demonstrated (not shown) and AFL was non‐inducible. The white circles indicate the area in the CTI ablated prior to creating the propagation/activation map.
Video clip 40.4 (a) A three‐dimensional propagation (voltage) map of typical AFL created using the Ensite™ balloon array electrode. Note the counterclockwise activation sequence around the tricuspid valve annulus in this patient. TV, tricuspid valve; CSO, coronary sinus ostium; IVC, inferior vena cava. (b) A three‐dimensional propagation (voltage) map following CTI ablation, during pacing from the low lateral right atrium using the Ensite™ balloon array electrode (same patient as in Video clip 40.2). Note the absence of lateral to medial conduction through the CTI with a strictly descending activation at the interatrial septum, confirming lateral to medial conduction block. However, typical AFL was still reinducible after CTI ablation. (c) A three‐dimensional propagation (voltage) map following CTI ablation, during pacing from the proximal coronary sinus using the Ensite™ balloon array electrode (same patient as in Video clip 40.2). This patient had typical AFL reinduced after CTI ablation. Note the delayed propagation through the CTI from medial to lateral, with narrowly spaced double potentials (right panel) recorded along the ablation line, indicating incomplete CTI block, resulting in slow conduction and reinducible typical AFL. Repeat ablation eliminated CTI conduction and rendered AFL non‐inducible.
Video clip 40.5 A Carto 3™ three‐dimensional propagation map (RAO view of right atrium) during atypical scar AFL. Note the double loop reentry pattern using an isthmus between areas of dense scar intermixed with viable atrial myocardium, across which a series of ablation lesions terminated AFL rendering it non‐inducible.
Video clip 41.1 Three‐dimensional reconstructed CT model (gray shell) of a patient who received mitral valve repair and biatrial cryoablation 2 years before electrophysiological study. At several (43) characteristic sites in the left atrium, post‐pacing interval (PPI) measurements were performed and the difference between PPI and the tachycardia cycle length (TCL) is color‐coded by using the LAT function of the three‐dimensional mapping system. Point by point the reentrant circuit is visualized: in this example the reentrant circuit involved the septal wall, the posterior wall in an oblique direction, and crossed between left atrial appendage (LAA) and left superior pulmonary vein (LSPV). The patient was successfully ablated by creating a linear lesion between the superior mitral annulus and the LSPV anterior to the LAA.
Video clip 41.2 Biatrial activation map using the Rhythmia system. Almost 15 000 electrograms were automatically annotated in a mapping time of less than 20 min using a 64‐electrode basket catheter. The active reentry circuit was affecting the right atrium; when reaching the interatrial connection, the passive activation of the entire left atrium can be nicely seen in this high‐resolution activation map.
Video clip 41.3 Three‐dimensional reconstructed CT with superimposed PPI map of a patient who presented with atypical atrial flutter and 2 : 1 conduction following pulmonary vein isolation 6 months previously. Spots where the post‐pacing return cycle equaled tachycardia cycle length are displayed in red. Greater differences are shown in yellow, blue and purple. The reentrant circuit involves the antrum of the left pulmonary veins, probably due to gaps in the circumferential lesions. Ablation of the anterior “ridge” close to the left atrial appendage terminated the tachycardia and made the patient non‐inducible after completion of reisolation of the pulmonary veins.
Video clip 80.1 A video of subxiphoid epicardial access in the AP projection. CS, His, RVA catheters were present along with an ICD RV lead. The ablation catheter entered the pericardial space via an inferior approach. Diaphragmatic staining along with free‐flowing contrast in the pericardial space was noted.
Video clip 80.2 A video of epicardial mapping in the RAO projection. CS, His, RVA catheters were present along with an ICD RV lead. The ablation catheter and sheath entered the pericardial space via an inferior approach and coursed laterally and superiorly over the left ventricle. The ablation catheter looped over the right ventricular free wall and positioned at the inferior interventricular septum. Coronary angiography showed the course of the right coronary artery (RCA) and the close proximity to the ablation catheter tip.
Video clip 83.1 Animation of MRI‐derived left ventricular signal intensity maps projected over color‐coded shells from endocardium to epicardium from a patient with healed inferior myocardial infarction. Tridimensional reconstruction of the aortic root is shown in purple. Paths of the border zone channels are highlighted with colored lines. Normal myocardium is represented in purple, core of the infarct in red, and border zone in...
| Erscheint lt. Verlag | 15.3.2019 |
|---|---|
| Sprache | englisch |
| Themenwelt | Medizin / Pharmazie ► Allgemeines / Lexika |
| Medizinische Fachgebiete ► Innere Medizin ► Kardiologie / Angiologie | |
| Medizin / Pharmazie ► Medizinische Fachgebiete ► Onkologie | |
| Schlagworte | Arrhythmias • Bildgebende Verfahren f. Herz- u. Gefäßuntersuchung • Cardiac CT Imaging • Cardiac Imaging • cardiac mapping • cardiac mapping techniques • cardiac morphology • Cardiac MRI • Cardiac Surgery • CARDIoGRAM • Cardiology • Cardiovascular Disease • cardiovascular imaging • clinical cardiology • CT scan • electroanatomical mapping • electro-anatomical mapping of the heart • electrophysiology • electrophysiology, cardiac electrophysiology • EP lab • Heart Arrhythmias • Heart health • heart mapping • heart mapping techniques • Kardiovaskuläre Erkrankung • Kardiovaskuläre Erkrankungen • mapping supraventricular tachyarrhythmias • mapping ventricular tachyarrhythmias • Medical Science • Medizin • MRI |
| ISBN-10 | 1-119-15261-5 / 1119152615 |
| ISBN-13 | 978-1-119-15261-3 / 9781119152613 |
| Informationen gemäß Produktsicherheitsverordnung (GPSR) | |
| Haben Sie eine Frage zum Produkt? |
Größe: 244,9 MB
Kopierschutz: Adobe-DRM
Adobe-DRM ist ein Kopierschutz, der das eBook vor Mißbrauch schützen soll. Dabei wird das eBook bereits beim Download auf Ihre persönliche Adobe-ID autorisiert. Lesen können Sie das eBook dann nur auf den Geräten, welche ebenfalls auf Ihre Adobe-ID registriert sind.
Details zum Adobe-DRM
Dateiformat: EPUB (Electronic Publication)
EPUB ist ein offener Standard für eBooks und eignet sich besonders zur Darstellung von Belletristik und Sachbüchern. Der Fließtext wird dynamisch an die Display- und Schriftgröße angepasst. Auch für mobile Lesegeräte ist EPUB daher gut geeignet.
Systemvoraussetzungen:
PC/Mac: Mit einem PC oder Mac können Sie dieses eBook lesen. Sie benötigen eine
eReader: Dieses eBook kann mit (fast) allen eBook-Readern gelesen werden. Mit dem amazon-Kindle ist es aber nicht kompatibel.
Smartphone/Tablet: Egal ob Apple oder Android, dieses eBook können Sie lesen. Sie benötigen eine
Geräteliste und zusätzliche Hinweise
Buying eBooks from abroad
For tax law reasons we can sell eBooks just within Germany and Switzerland. Regrettably we cannot fulfill eBook-orders from other countries.
aus dem Bereich