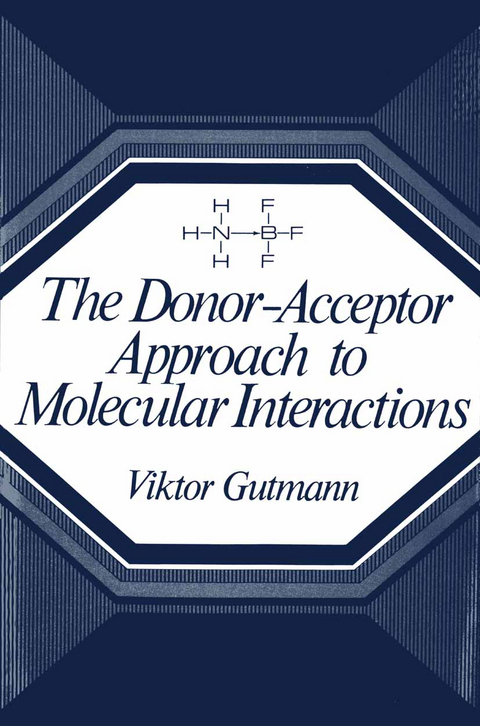
The Donor-Acceptor Approach to Molecular Interactions
Springer-Verlag New York Inc.
978-1-4615-8827-6 (ISBN)
1. Basic Considerations.- 1.1. Introduction.- 1.2. Charge-Density Rearrangement Caused by an Intermolecular Interaction.- 1.3. The First Bond-Length Variation Rule.- 1.4. The Second Bond-Length Variation Rule.- 1.5. The Third Bond-Length Variation Rule.- 1.6. General Aspects.- 2. Empirical Parameters for Donor and Acceptor Properties.- 2.1. Solvent Characterization by Empirical Parameters.- 2.2. The Donor Number.- 2.3. The Acceptor Number.- 3. Molecular Adducts.- 3.1. Theoretical Approaches.- 3.2. Application of the Bond-Length Variation Rules.- 3.3. Application of the Empirical Parameters.- 3.4. Metal Carbonyls and Related Compounds.- 3.5. Molecular Adducts of Organic Carbonyl Compounds and of Phenols.- 4. Bond-Length Considerations in the Crystalline State.- 4.1. Molecular Lattices.- 4.2. Ionic Crystals.- 4.3. Silicates.- 4.4. Bond-Length Variability in Complex Compounds.- 4.5. Bond-Length Variations under Pressure.- 5. Interface Phenomena.- 5.1. Lattice Contraction at Clean Crystal Surfaces.- 5.2. Crystal Size and Lattice Deformation.- 5.3. Adsorption Phenomena.- 5.4. Surface Layers.- 5.5. Epitaxy Phenomena.- 5.6. Solid-Water Interfaces.- 5.7. Other Surface Phenomena.- 5.8. Crystal Growth.- 6. Molecular Association in the Liquid State.- 6.1. Association by Hydrogen Bonds.- 6.2. Liquid Water and Its Solutions.- 6.3. Association in Aprotic Liquids.- 7. Ion Solvation.- 7.1. General Considerations.- 7.2. Relationships between Thermodynamic Solvent Transfer Quantities and Empirical Solvent Parameters.- 7.3. Charge-Density Rearrangement by Ion Solvation.- 7.4. Outer-Sphere Effects in Cation Solvation.- 7.5. Outer-Sphere Effects in Anion Solvation.- 8. Redox Properties.- 8.1. Solvent Effects.- 8.2. Ligand Effects.- 9. Solvation in Solvent Mixtures.- 9.1. Preferential Solvation.- 9.2. Solvent-Solvent Interactions.- 9.3. Homoselective and Heteroselective Solvation.- 10. Ionization Equilibria.- 10.1. Heterolysis by Coordinating Interactions.- 10.2. Heterolysis by Redox Interactions.- 10.3. Homolytic and Heterolytic Dissociation Energy.- 10.4. Solvent-Separated Ion Pairs: The Role of the Dielectric Constant.- 10.5. Outer-Sphere Ion Association.- 10.6. The Concept of Contact Ion Pairs.- 10.7. A Simplified Reaction Scheme.- 11. Complex Stabilities in Solution.- 11.1. Qualitative Considerations.- 11.2. Quantitative Considerations.- 11.3. Autocomplex Formation.- 11.4. Solubility Considerations.- 12. Kinetics of Substitution Reactions.- 12.1. General Considerations.- 12.2. Substitution at Carbon.- 12.3. Substitution at Metal Complexes.- 12.4. Outer-Sphere Effects.- 13. Kinetics of Redox Reactions of Metal Complexes.- 13.1. General Considerations.- 13.2. Outer-Sphere Mechanism.- 13.3. Inner-Sphere Mechanism.- 14. Solvent and Reaction Effects on the Course of Substitution and Insertion Reactions.- 14.1. General Considerations.- 14.2. Reactions Involving Organo-Alkali Metal Compounds.- 14.3. Grignard Reactions.- 14.4. Reactions of Carbon Dioxide.- 14.5. Reactions of Molecular Nitrogen.- 14.6. Reactions of Silicon Compounds.- 14.7. Reactions of Boron Compounds.- 15. Miscellaneous Applications.- 15.1. Outer-Sphere Effects on Oxo Anions.- 15.2. Molten Salts.- 15.3. Liquid-Liquid Extraction.- 15.4. Stabilization of Colloids in Liquid Dispersion Media.- 15.5. Solvent Effects on Electronic Spectra.- 16. Homogeneous and Heterogeneous Catalysis.- 16.1. Catalytic Ability.- 16.2. Catalytic Selectivity in Homogeneous Catalysis.- 16.3. Catalytic Selectivity in Heterogeneous Catalysis.- 16.4. Elimination Reactions on Solid Catalysts.- 16.5. Addition of Hydrogen Halides to Alkenes on Solid Catalysts.- 17. Biochemical Applications.- 17.1. General Considerations.- 17.2. Remarks on the Role of Water in Biological Systems.- 17.3. Oxidation of Hemoglobin.- 17.4. Carbonic Anhydrase.- 17.5. Corrinoids.- 17.6. Activation of Phosphate Transfer.- References.
| Erscheint lt. Verlag | 28.1.2012 |
|---|---|
| Zusatzinfo | XVI, 279 p. |
| Verlagsort | New York, NY |
| Sprache | englisch |
| Maße | 152 x 229 mm |
| Themenwelt | Naturwissenschaften ► Chemie ► Anorganische Chemie |
| ISBN-10 | 1-4615-8827-8 / 1461588278 |
| ISBN-13 | 978-1-4615-8827-6 / 9781461588276 |
| Zustand | Neuware |
| Haben Sie eine Frage zum Produkt? |
aus dem Bereich


